
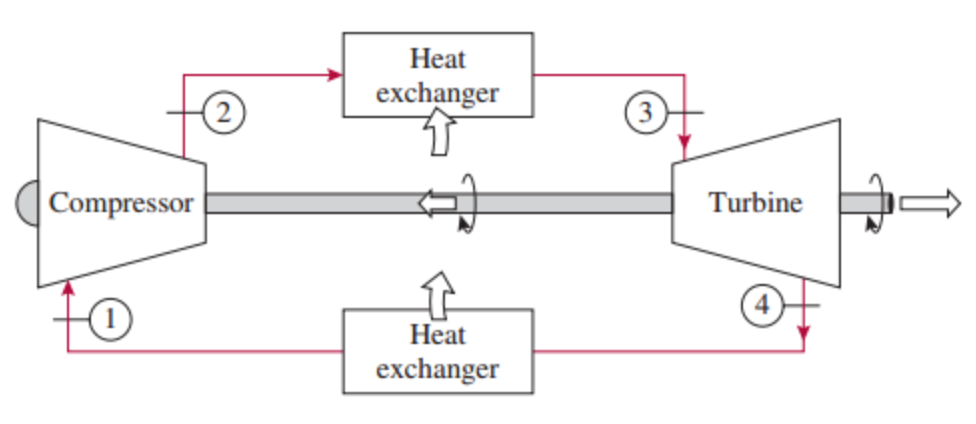

Positive value of work interaction indicates work done by the system and negative value of work interaction indicates work done on the system. Note: Negative value of heat interaction indicates heat rejected by the system and positive value of heat interaction indicates heat added to the system. Since it is a heat rejection process, temperature of the gas decreases.īelow is the table which shows heat and work interactions of the gas turbine, along with the change in the internal energy. A little decrease in volume happens due to heat rejection. Below are P-V and T-S Diagrams of the Brayton (or Joule) Cycle. Brayton cycle is named after George Brayton, an American engineer who developed it. The presented work analyses the design space and performance potential of microfabricated Brayton cycle and Rankine cycle devices, accounting for lower component efficiencies, temperatures limited. Gas turbines are used to generate power at many places. Since it is an expansion process, volume of the gas increases. Brayton cycle (or Joule Cycle) is a thermodynamic cycle upon which a Gas turbine works. Here a little dip in temperature occurs due to expansion. Since it is a heat addition process, temperature of the gas increases. A little increase in volume happens due to heat addition. Since it is a compression process, volume of the gas decreases. Here a little rise in the temperature of gas occurs due to compression. Brayton cycle is named after George Brayton, an American engineer who developed it.īelow are P-V and T-S Diagrams of the Brayton (or Joule) Cycle.īrayton Cycle is comprised of four processes Not included in $W_S$ is the work required to push working fluid into- and out of the device.Brayton cycle (or Joule Cycle) is a thermodynamic cycle upon which a Gas turbine works. $$\dot$ is the mass flow rate through the compressor or turbine, $\Delta h$ is the change in enthalpy per unit mass of the working fluid in passing through the device, and $W_S$ is the so-called "shaft work." This is not the total amount of work, but only the part of the work delivered to- or derived from the rotating shaft. For this monatomic gas, find the work done in each step. Describe the types of expansions and compressions that are a part of this engine cycle. To run this Brayton cycle, go through the steps in order. The net cycle work done is the area enclosed by the cycle on the P-v diagram. This, then, gives the ideal gas law as PV NT. That said, for a compressor or turbine operating adiabatically at steady state, the open system version of the first law of thermodynamics tells us that: The Carnot cycle was introduced in Chapter 5 as the most efficient heat.
NET WORKDONE IN BRAYTON CYCLE HOW TO
For an engineer, understanding the relationship between the closed system version of the first law and the open system version (and how to apply the latter) is vital (in my judgment), particularly when dealing with power cycles such as in your present problem. You said that you want to understand the fundamentals and don't want to memorize stuff. So your first step is to go back to your textbook and get an understanding of this derivation. It requires too extensive a derivation if you are unfamiliar with the open system version of the first law of thermodynamics for a system operating at steady state.


 0 kommentar(er)
0 kommentar(er)
